
Table of Contents
Introduction:
- HACCP stands for Hazard Analysis and Critical Control Point.
- Originally developed in the 1960’s by NASA and a group of food safety specialists.
- According to US Food and Drug Administration, “HACCP is a management system in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product”.
- HACCP is designed for use in all segments of the food industry from growing, harvesting, processing, manufacturing, distributing, and merchandising to preparing food for consumption.
- It is the means of securing food safety from harvesting to consumption.
- Tool to identify the hazards and applying the major for the food safety.
- HACCP can be applied in every step of food processing.
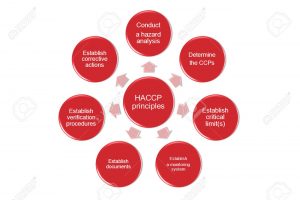
Principles of HACCP:
Principle 1: Conduct a hazard analysis.
Principle 2: Determine the critical control points (CCPs).
Principle 3: Establish critical limits.
Principle 4: Establish monitoring procedures.
Principle 5: Establish corrective actions.
Principle 6: Establish verification procedures.
Principle 7: Establish record-keeping and documentation procedures.
1. Conduct a hazard analysis:
- Hazard analysis is the very first step.
- All the potential hazards are identified in this step
- The hazard is defined as the any physical, chemical and biological agent that possess the possible risk or threat to the health.
- The basic physical hazards are metal contamination, presence of inedible items. Chemical hazards include the presence of toxins or any unwanted chemicals and biological hazard denote the presence of pathogens.
- Any method that can control these hazards needs to be adopted.
2. Determine the critical control points (CCPs):
- CCP stands for critical control point.
- At this step the control measures can be applied.
- Determination of CCP refers to identifying the point at which the control measures can be applied to eliminate the hazard that has been previously identified.
- CCP is essential to prevent or eliminate hazard or to reduce it to an acceptable level.
- Examples of CCPs may include: Cooking, chilling, metal detection, setting into suitable temperature.
3. Establish critical limits:
- Once the CCP is identified, critical limit points needs to be assigned for each CCP.
- There can be one or more critical limits for each CCP
- Critical limits are defined as the maximum and/or minimum value of CCP such that the hazards are controlled and food safety is assured.
- Critical limits must be defined on the scientific grounds and basis
- Critical limits are usually based on factors such as: temperature, time, physical dimensions, humidity, moisture level, water activity (aw), pH, regulatory levels, etc.
4. Establish monitoring procedures
- Generally, refers to the planning and carrying out monitoring for CCP
- Monitoring should be done on regular basis
- Monitoring technique may differ based on the CCP
- Monitoring is necessary to identify the deviation and apply the effective measures
- Monitoring is also necessary for future purpose and verification as monitoring process is documented.
5. Establish corrective actions
- Corrective action is taken when the critical limit is not met
- Corrective actions are pre-decided for each CCP
- These actions make sure that no any harmful product reaches the market for consumption
6. Establish verification procedures
- HACCP plan must be validated.
- For testing the validity of the plan several steps can be taken such as checking out the random samples, reviewing the process, confirming that the CCP are under control.
- Verification activities can be carried out by the external hired officers or the internal members.
- No matter who performs the verification, it should be unbiased and fairly carried out.
7. Establish record-keeping and documentation procedures
- Record keeping is must in HACCP
- Records maintained should have the records or information regarding HACCP plan, CCP, critical limits, monitoring, corrective action, all the procedures including the verification procedures.
- Recording keeping is necessary of validation and proper application of HACCP.
Benefits of HACCP:
- Ensures the consumer regarding the safety of the product
- Prioritizes food safety and works to eliminate any kind of hazard
- Necessary for the consistent quality products
- Provides the framework to produce foods safely and to prove they were produced safely.
- Prevents from the possible health outcomes that could have occurred due to mishandling during food production steps
- HACCP is also necessary for obtaining validation.
References and for more information:
https://www.22000-tools.com/what-is-haccp.html
https://www.fda.gov/food/guidanceregulation/haccp/ucm2006801.htm
http://www.fao.org/docrep/005/Y1579E/y1579e03.htm
https://www.foodsafety.com.au/resources/articles/everything-you-need-to-know-about-haccp
https://myhaccp.food.gov.uk/help/guidance/principle-2-determine-critical-control-points-ccps
https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/afs4341
https://myhaccp.food.gov.uk/help/guidance/principle-3-establish-critical-limits
https://pdfs.semanticscholar.org/52e1/294fe93be529140014f808a352d4d4af50c1.pdf
https://www.mhlw.go.jp/english/topics/importedfoods/guideline/dl/05.pdf
https://agrowhizz.wordpress.com/2017/02/23/what-is-the-importance-of-haccp/
http://slbs.org.lc/document_file/HACCP_Brochure_current.pdf
http://www.fao.org/docrep/005/Y1579E/y1579e03.htm
https://www.fda.gov/Food/GuidanceRegulation/HACCP/
https://www.fda.gov/food/guidanceregulation/haccp/ucm2006801.htm#princ